Dose discontinuation
Fewer patients receiving BRAFTOVI + cetuximab discontinued therapy due to adverse events compared to control arm*
Rate of discontinuation primarily due to AEs (any grade) per updated post-hoc analysis 13
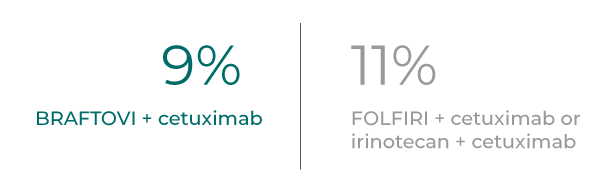
- In patients treated with BRAFTOVI + cetuximab, the rate of all study drug discontinuation due to any adverse reaction was 1.9%1


