Tumor Response
The majority of patients with measurable disease experienced tumour shrinkage with BRAFTOVI + cetuximab vs control arm*
Best percentage change in tumour response per primary analysis3,a
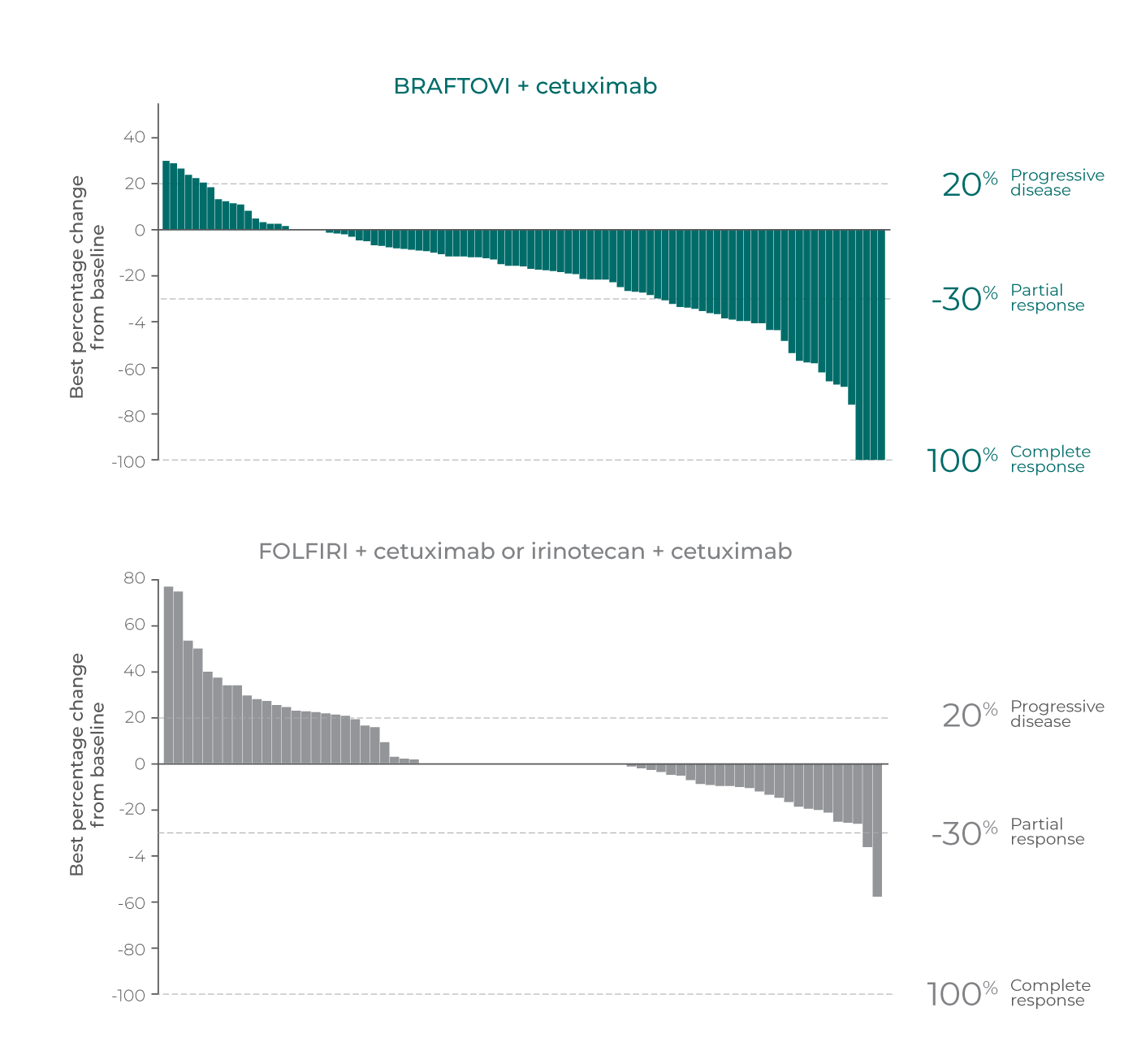
Reprinted with permission from Massachusetts Medical Society.
- The sum of longest diameter change includes patients with measurable disease per RECIST v1.1 with a baseline and at least 1 post-baseline scan. Only patients with measurable disease are included in the above waterfall plots3,8


