Overall Survival
BRAFTOVI + cetuximab significantly improved median overall survival vs control arm*
Median overall survival was at least 3 months longer with BRAFTOVI + cetuximab vs control arm in both analyses1
Overall survival per updated post-hoc analysis (median follow-up 12.8 months)13
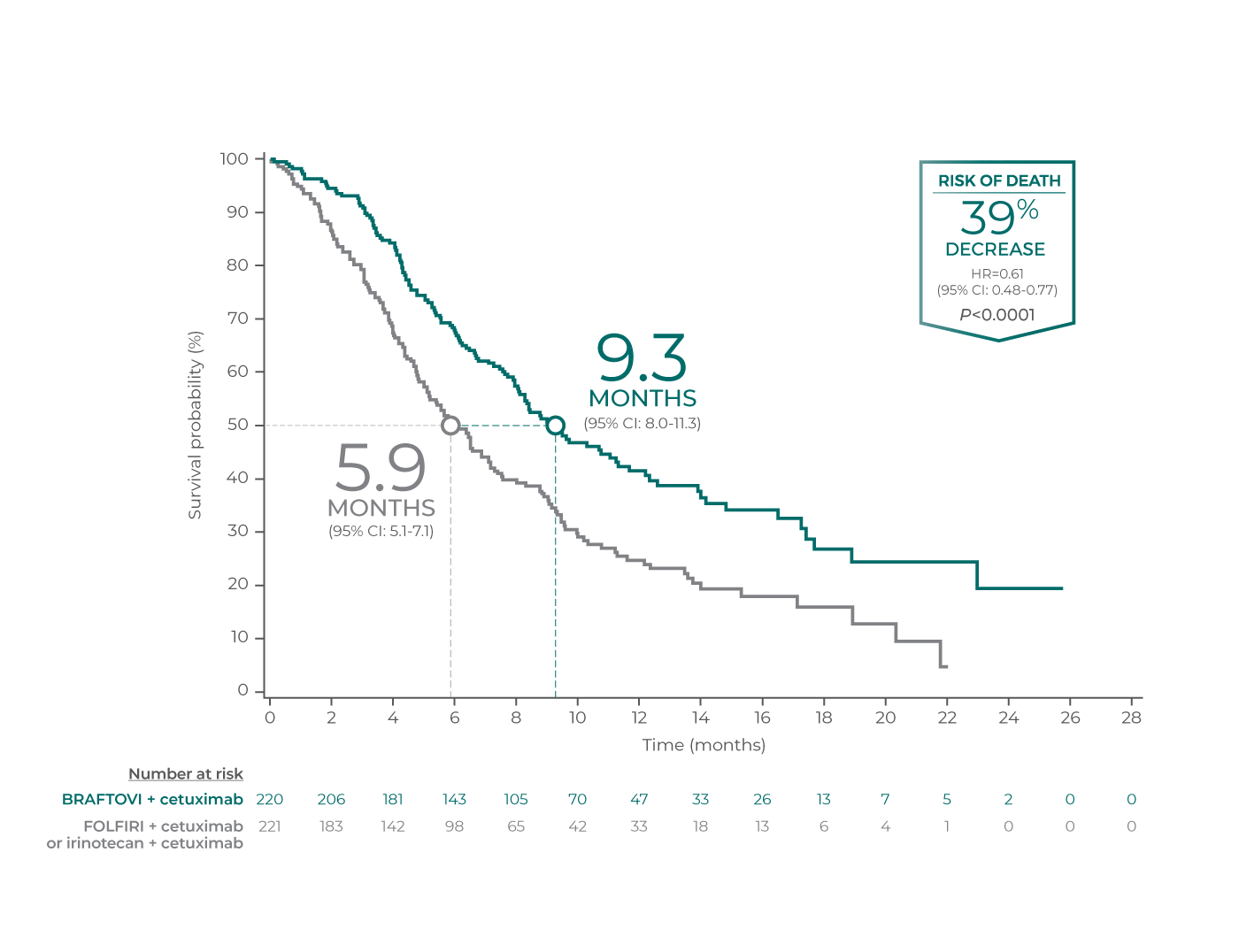
Overall survival per primary analysis3
- 8.4 months (95% CI: 7.5-11.0) with BRAFTOVI + cetuximab (n=220) vs 5.4 months (95% CI: 4.8-6.6) with FOLFIRI + cetuximab or irinotecan + cetuximab (n=221) (HR=0.60 [95% CI: 0.41-0.88], P=0.0002)


