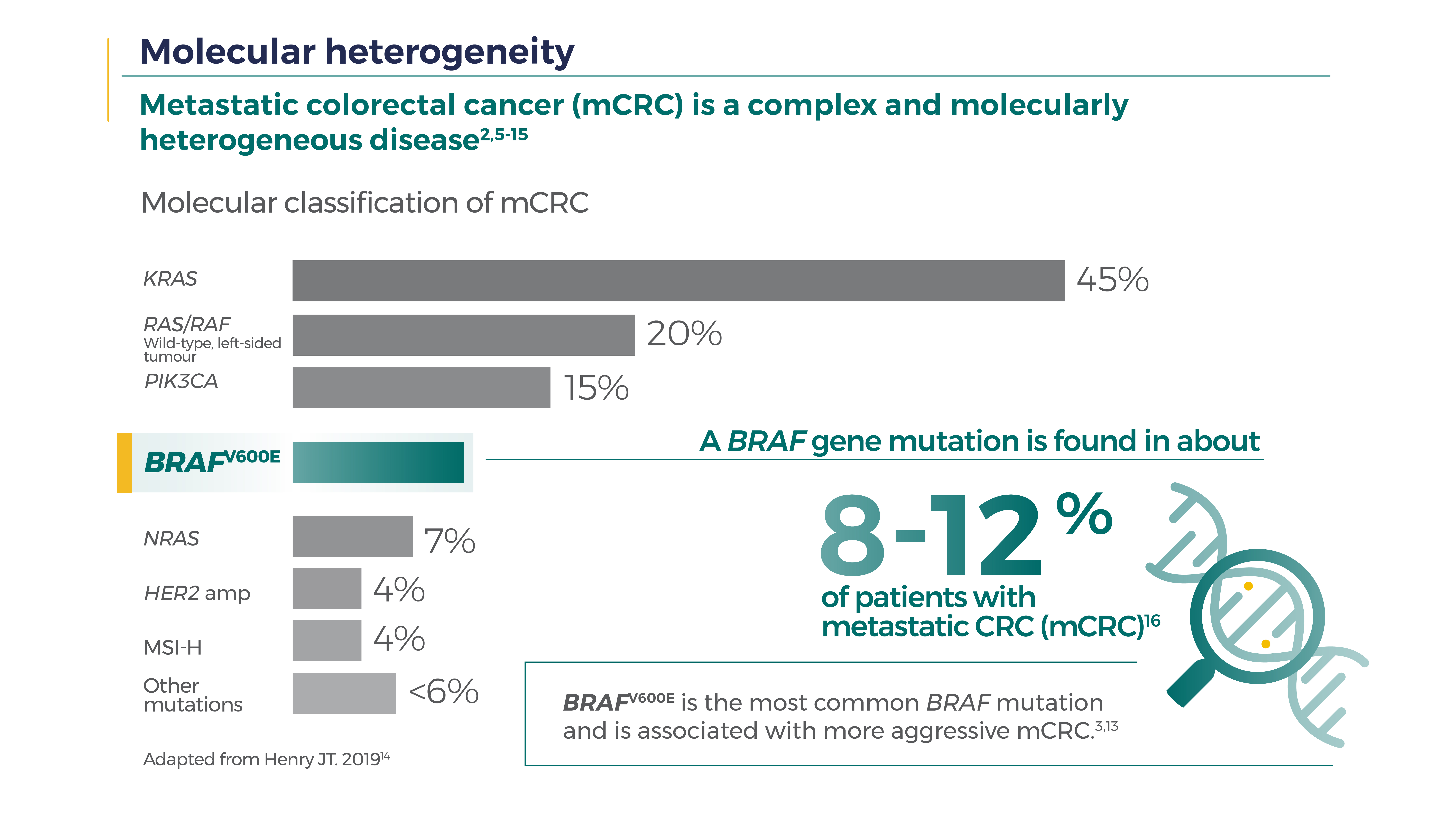
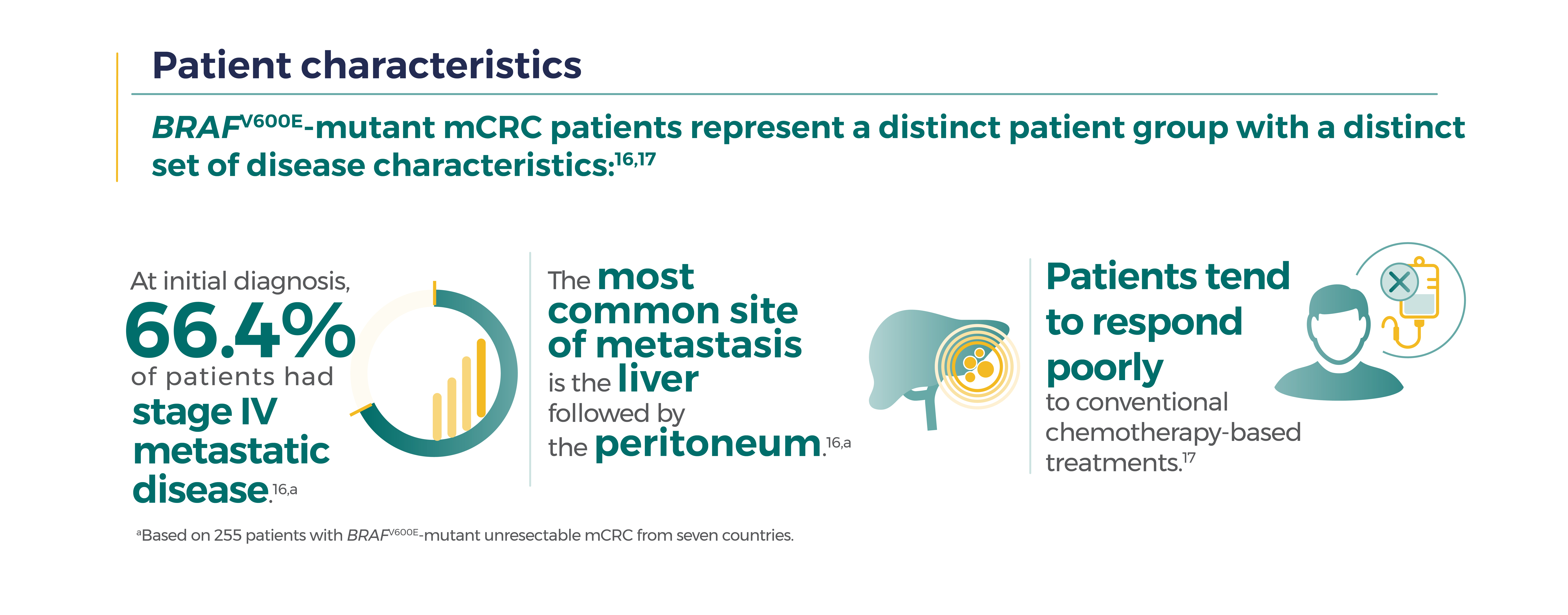
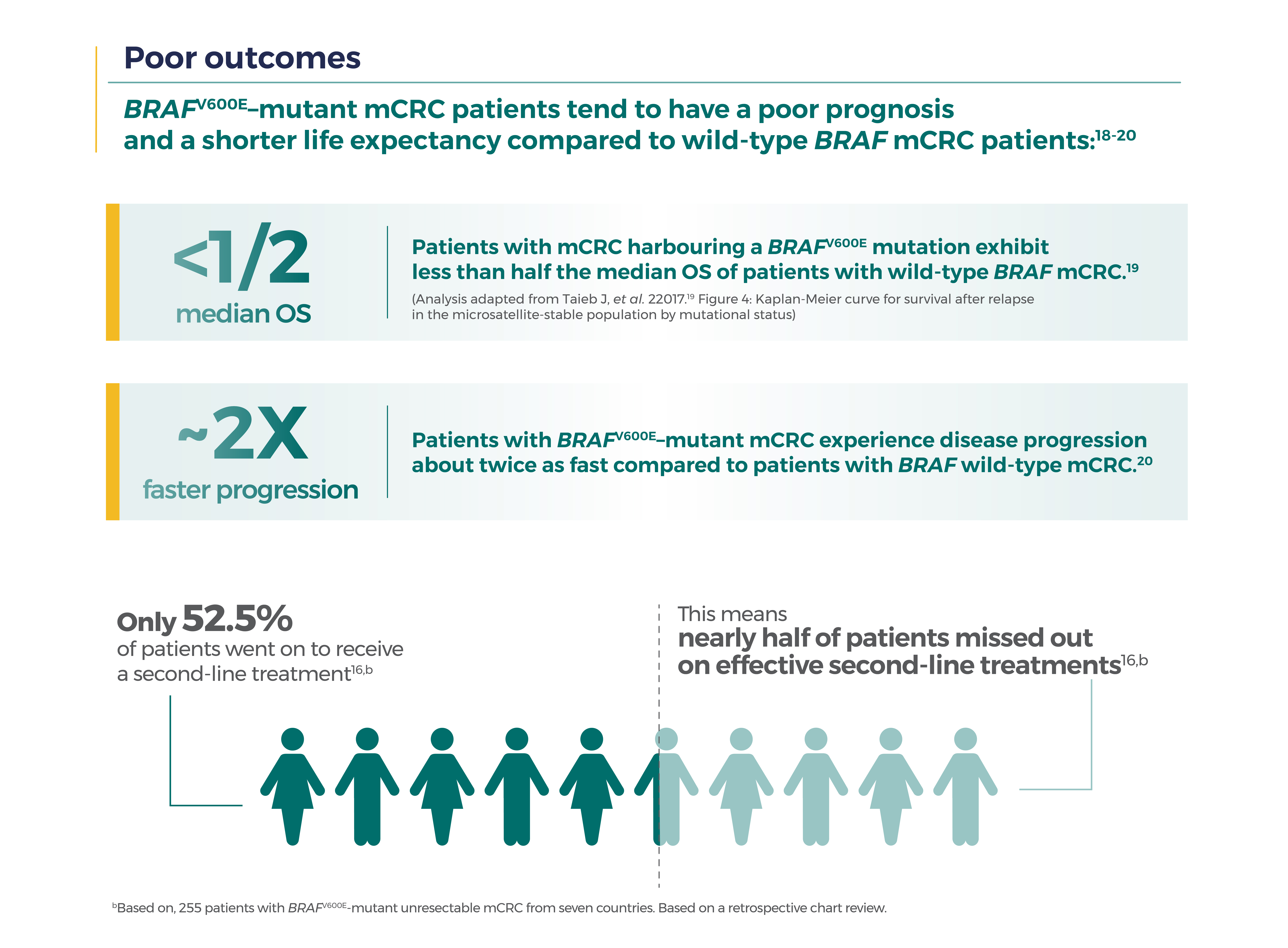
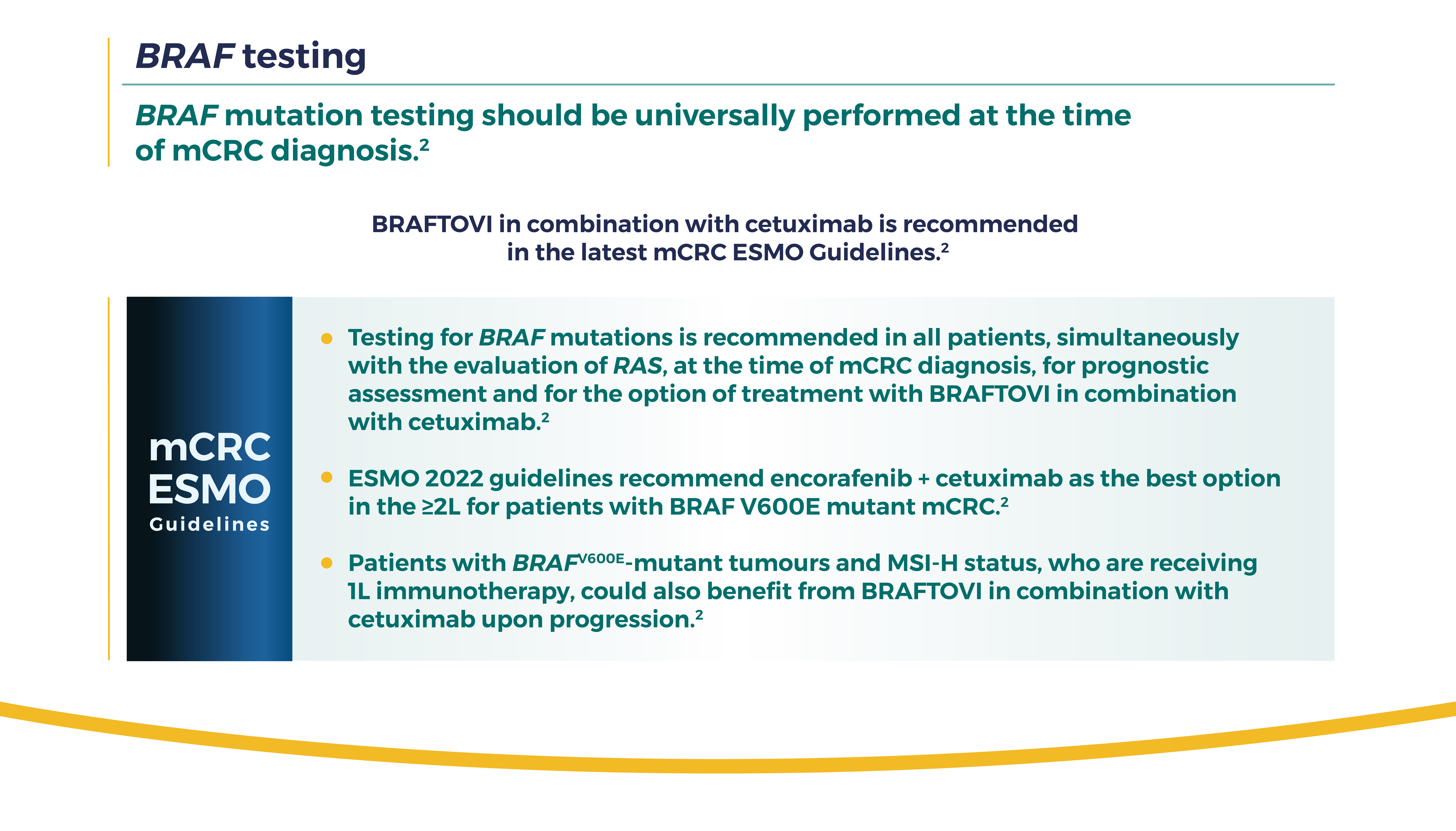
BRAF V600E-mutant mCRC
The first and only treatment approved specifically for patients with BRAF V600E-mutant mCRC, who have received prior systemic therapy1,2
BRAFV600E-mutant mCRC
is an area of high unmet need2-13